Exploiting Specific Interactions toward Next-Generation Polymeric Drug Transporters
Sebastian Wieczorek,a Eberhard Krause,b Steffen Hackbarth,c Beate Röder,c Anna K. H. Hirsch,d and Hans G. Börner*,a
a Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, D-12489 Berlin, Germany
b Leibniz-Institut für Molekulare Pharmakologie (FMP), Robert-Rössle-Strasse 10, D-13125 Berlin, Germany
c Department of Physics, Humboldt-Universität zu Berlin, Newton Strasse 15, D-12489 Berlin, Germany
d Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
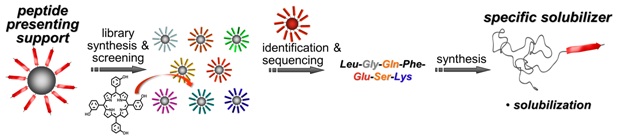
A fluorescence-based screening method was established to assist the design of peptide block poly(ethylene oxide) copolymers to specifically host the photosensitizer m tetra(hydroxyphenyl)chlorin. Solubilization capacity (maximum payload of m-THPC in the bioconjugates) and release kinetics from the transport system proved to depend strongly on the peptide sequence of the peptide-PEO conjugates. Most favorable for a photosensitizer transport system, an effective solubilization process led to micellar solubilized drugs, which exhibit a silent transport state with very low or no activity, as indicated by direct singlet-oxygen-generation measurements. Depending on the peptide-binding domain, the transporters release the cargo to serum albumin protein. Tunable release profiles can be programmed in the peptide segment, which is an improvement over established polymeric solubilizers. Solubility, dispersibility, and compatibility of small functional compounds appear to be problematic not only in biomedicine but also in fields ranging from demixing of UV stabilizers in polymers to incompatibility of additives in engine lubricants to surface remodulation in coatings. It is foreseeable that specific hosting of active compounds can be of advantage beyond drug transporters.
|