|
|

|
|
Prof. Dr. habil. Hans Börner
| Phone: |
+49 (0)30 2093-7348 |
| Fax: |
+49 (0)30 2093-7500 |
Email
|
|
|
|
|
|
|
|
Contact Us |
|
|
|
Laboratory for Organic Synthesis of Functional Systems
Department of Chemistry
Humboldt-Universität zu Berlin
Brook-Taylor-Str. 2
12489 Berlin
Germany
Sekretariat
Phone: +49 (0)30 2093-7349
Fax: +49 (0)30 2093-7215
Room: 0'144
Email
office.functional-systems hu-berlin.de |
|
|
|
|
|
|
Welcome to BörnerLab

Research Overview
- Synthesis and design of functional hybrid polymers (bioconjugates)
- Bio-mimetic formation of structure and function in synthetic polymers (peptide-guided organization and structure based functions)
- Pseudopeptides and precision polymers for biomedical applications (integrated polymer systems for gene or drug delivery)
- Bio-functionalization of surfaces (bioactive polymer fibers, scaffolds and material interfaces; Bio-inspired adhesion segments in block copolymers, (bio)-functional coatings, crystal growth modifiers)
Objectives: Controlling interactions in synthetic polymers as precisely as in proteins would have a strong impact on polymer science. Advanced structural and functional control can lead to rational design of, integrated nano- and microstructures. To achieve this, properties of oligopeptides were exploited. By incorporating these as monodisperse segments into synthetic polymers we show how to program structure formation in polymers, control inorganic-organic interfaces in fiber composites, induce structure in biomacromolecules for biomedical applications and generate bioactive surfaces to control biological systems.
|
News
|
26.02.2026 - Gratulation an Keven Walter zum Promotionsabschluss mit summa cum laude!

Gratulation Keven Walter zum Abschluss der Promoten mit höchster Auszeichnung summa cum laude. Phantastische Resultate von neuen Monomeren bis zum Anwendungsdemonstrator. Debonding on command gets green and reality
|
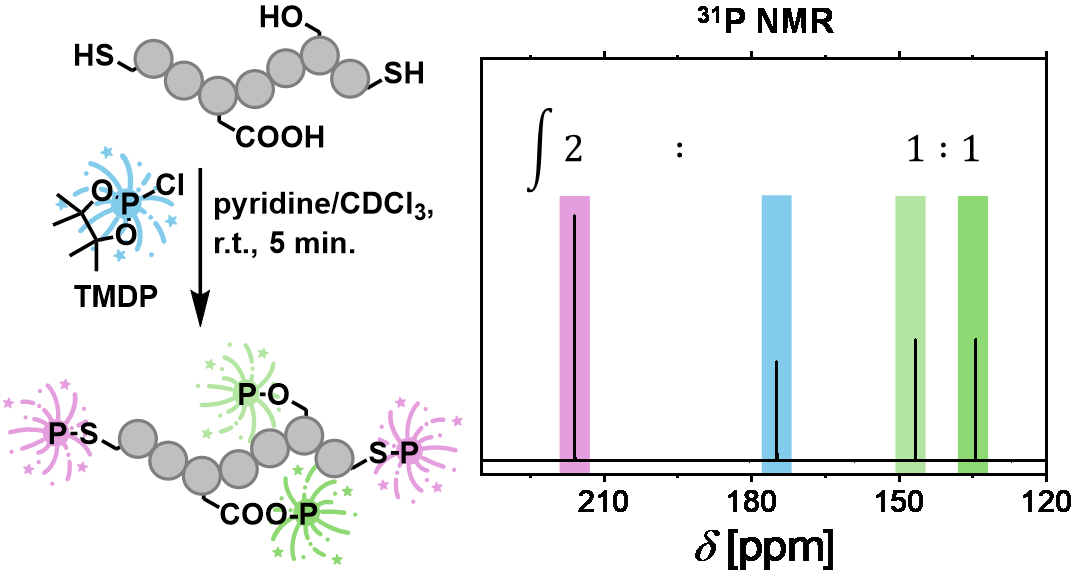
06.01.2026 - Advancing Quantitative 31P NMR Spectroscopy for Reliable Thiol Group Analysis
New paper released in ACS Macro Letters
A straightforward 31P NMR spectroscopy protocol based on TMDP phosphitylation enables reliable thiol quantification in both small molecules and polymers. At the same time, the method allows the detection of hydroxy and carboxylate by-products, providing a rapid and comprehensive assessment of thiol-based (macro)molecules and their functional group composition.
DOI: https://pubs.acs.org/doi/10.1021/acsmacrolett.5c00739
|
21.11.2025 - Gratulation an Lukas Bangert zum erfolgreichen Promotionsabschluss!

Magna cum laude
Toller Endspurt und starke Methode für Papierkomposite!
|
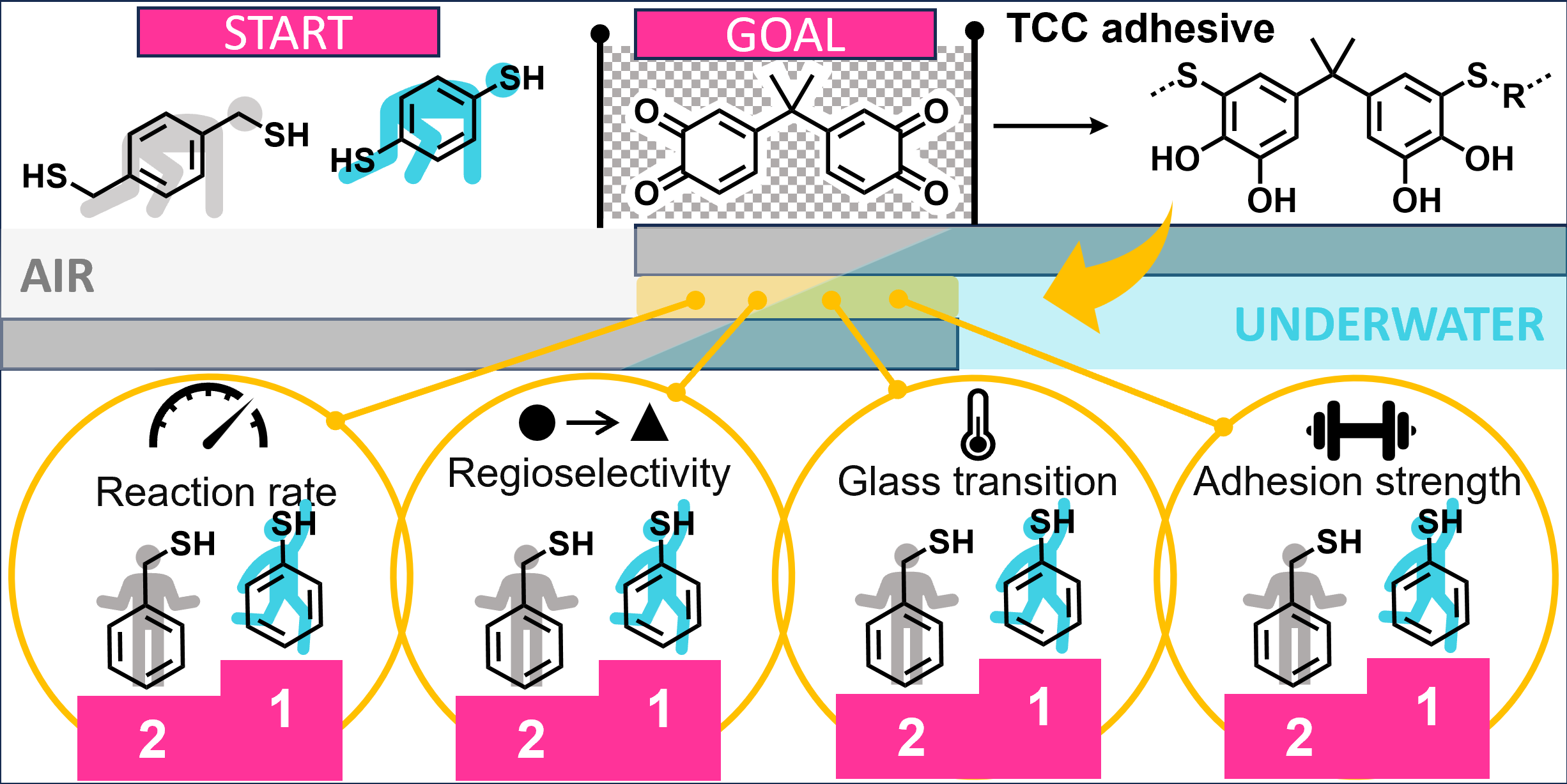
31.10.2025 - New paper released in Polymer Chemistry!
Aromatic phenolic dithiols open new possibilities for bioinspired adhesives, reacting fast and cleanly with bisquinone A and producing polymers that maintain strong bonds even underwater, offering a path toward next-generation performance adhesives inspired by nature.
Full article link: https://doi.org/10.1039/D5PY00709G
|
31.10.2025 - New Paper Alert! TCC Polymers for Non-Covalent Paper Reinforcement
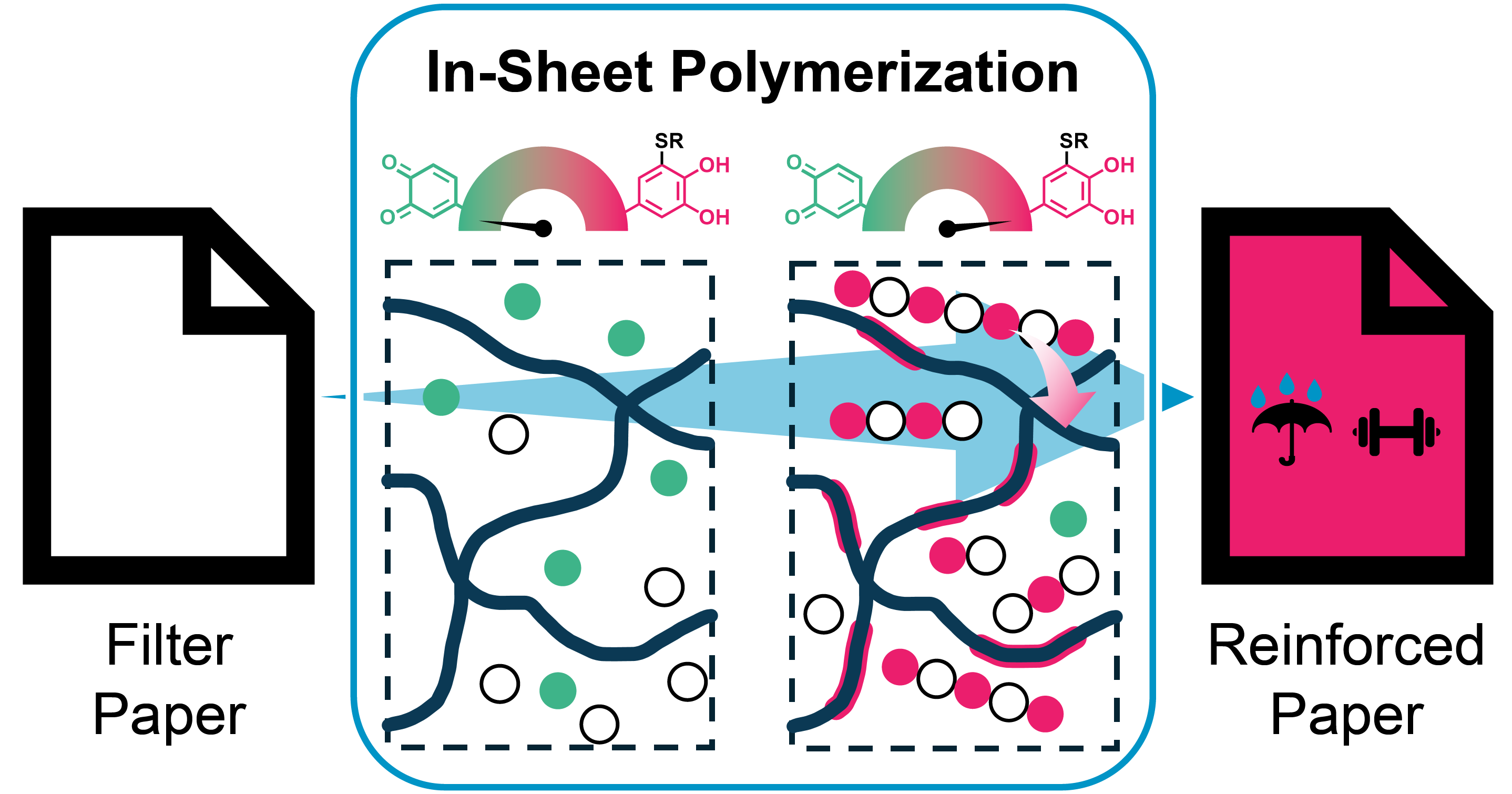
The adhesiveness and rigidity of aromatic TCC-polymers have been harnessed in the fabrication of purely non-covalent paper-polymer-composites. Using an in-sheet polymerization & adhesion (InSPA) approach, paper fibers could be homogeneously infused with hydrophobic polymers, resulting in significant mechanical reinforcement and water-repellent behavior. In contrast to commercial reinforcement agents, the coating could be fully extracted in a simple aqueous washing procedure, allowing for straightforward recycling of the underlying cellulose fibers.
DOI: https://doi.org/10.1021/acsami.5c13781
|
21.10.2025 - Honored Among Berlin’s Top 100 Scientists
I am honored to be included in Der Tagesspiegel's selection of "Die 100 wichtigsten Köpfe der Berliner Wissenschaft."
This recognition reflects not only individual research accomplishments but also the collective excellence of our research group, @BörnerLab @HU Berlin, whose work is driven by curiosity, scientific collaboration, and societal impact.
Both Berlin and Humboldt-Universität zu Berlin continue to stand as powerhouses of innovation, and it is a privilege to contribute to this research environment by developing advanced materials concepts for future technological applications.
|
17.06.2025 - ERC Advanced Grant
.jpg) |
Prof. Dr. Hans Börner wurde für sein Projekt IDefix mit einem ERC Advance Grant in Höhe von 2,5 Millionen geehrt und kann nun mit seinem interdisziplinären Team „Tiefe Sequenzdaten-analyse elektrochemisch schaltbarer Peptide nutzen, um Wege zu neuartigen Polymerkleb- und Funktionsstoffen“ zu erforschen. |
|
26.05.2025 - Congratulations to Dr. Steffen Busche!

We warmly congratulate Steffen Busche on successfully defending his PhD thesis "A Modular System for Personalized Support Materials by Inverse Electron Demand Diels-Alder Reaction of Norbornenes and 1,2,4,5-Tetrazines". A great achievement—congratulations, Dr. Busche!
|
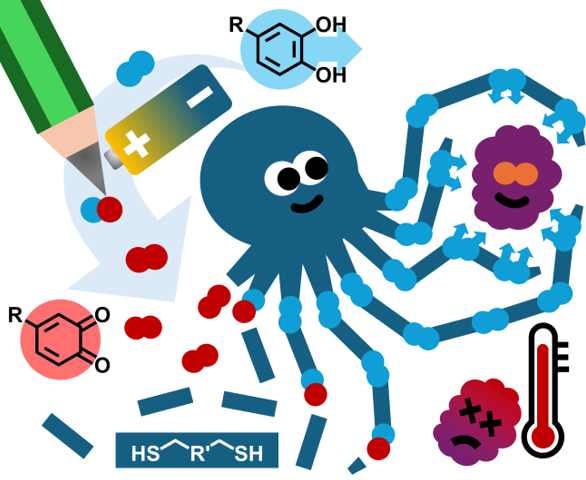
15.01.2025 - Electrosynthesis of mussel-inspired adhesive polymers as a novel class of transient enzyme stabilizers
New paper published in Angewandte Chemie by the electraDetouch team.
The electrochemical oxidation of multifunctional catechols represents a “green” and clean alternative pathway to ortho-quinones, which are used to form oligomer networks with branched multi-thiols. In these networks, the catechols are restored and represent adhesive points, which can establish transient interactions with enzymes. These oligomer/enzyme-conjugates are shielding the enzymes from heat stress, thus preventing denaturation. (https://doi.org/10.1002/anie.202419684)
|
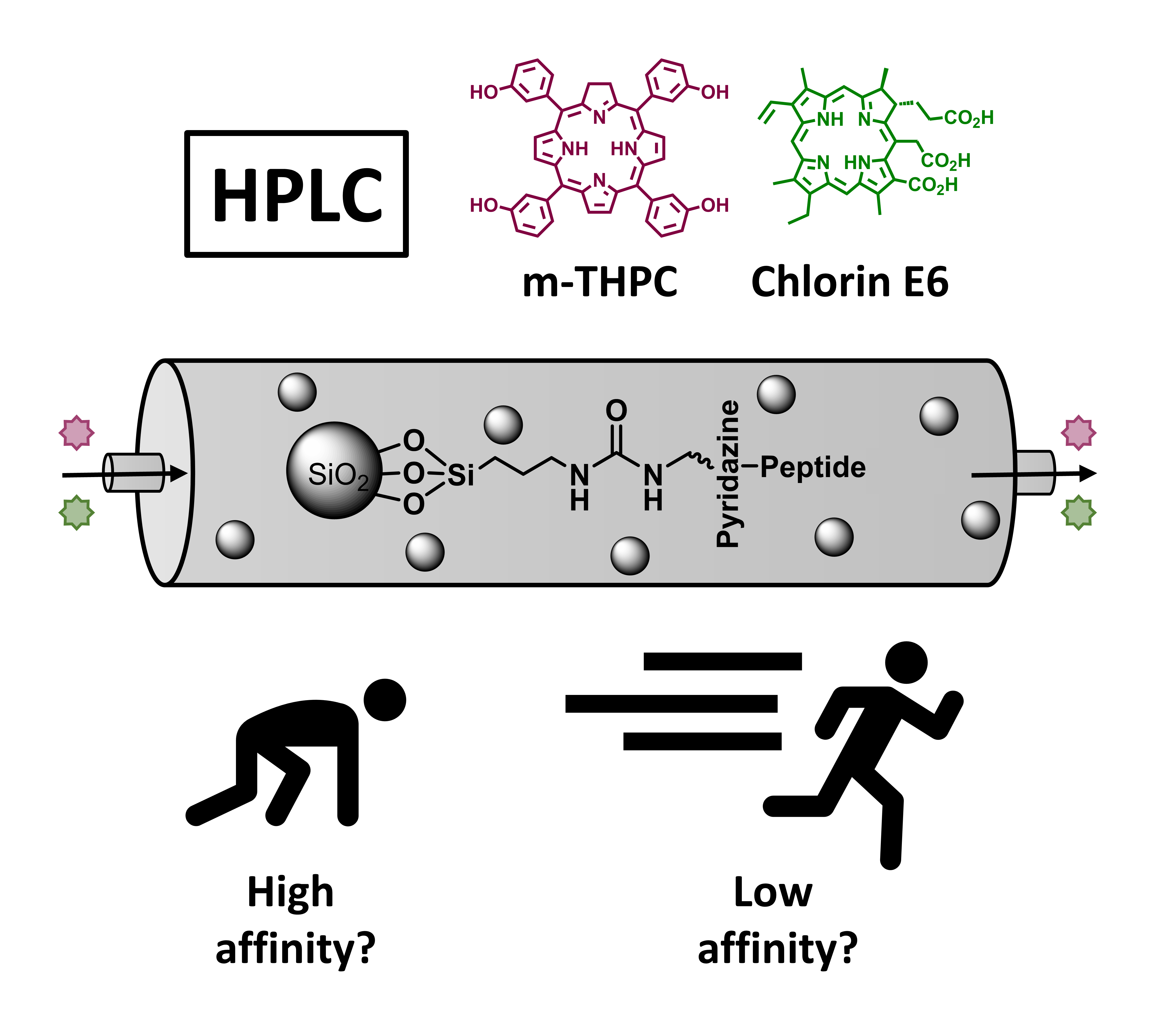
06.01.2025 - Toward Personalized Stationary Chromatography Phases: Equipping Commercial Silica Phases with Selected Peptide-Based Binding Domains to Tailor Affinity
The last paper of Steffen Busche was released in Advanced Materials Interfaces.
The 7-mer peptide motifs QFFLFFQ, QFFEFFQ and QFQQSFF, known as good, medium and poor binders for the photosensitizers m-THPC and Chlorin E6 are covalently immobilized on commercial amino-functionalized silica particles by inverse electron-demand Diels-Alder ligation. After proof of compound binding by UV/Vis batch experiments, professionally packed HPLC columns show compound specific separation performance with clear differences in retention times.
|
| show all news |
|
|







.jpg)