|
|

|
|
Prof. Dr. habil. Hans Börner
| Phone: |
+49 (0)30 2093-7348 |
| Fax: |
+49 (0)30 2093-7500 |
Email
|
|
|
|
|
|
|
|
Contact Us |
|
|
|
Laboratory for Organic Synthesis of Functional Systems
Department of Chemistry
Humboldt-Universität zu Berlin
Brook-Taylor-Str. 2
12489 Berlin
Germany
Sekretariat
Phone: +49 (0)30 2093-7349
Fax: +49 (0)30 2093-7215
Room: 0'144
Email
office.functional-systems hu-berlin.de |
|
|
|
|
|
|
Welcome to BörnerLab

Research Overview
- Synthesis and design of functional hybrid polymers (bioconjugates)
- Bio-mimetic formation of structure and function in synthetic polymers (peptide-guided organization and structure based functions)
- Pseudopeptides and precision polymers for biomedical applications (integrated polymer systems for gene or drug delivery)
- Bio-functionalization of surfaces (bioactive polymer fibers, scaffolds and material interfaces; Bio-inspired adhesion segments in block copolymers, (bio)-functional coatings, crystal growth modifiers)
Objectives: Controlling interactions in synthetic polymers as precisely as in proteins would have a strong impact on polymer science. Advanced structural and functional control can lead to rational design of, integrated nano- and microstructures. To achieve this, properties of oligopeptides were exploited. By incorporating these as monodisperse segments into synthetic polymers we show how to program structure formation in polymers, control inorganic-organic interfaces in fiber composites, induce structure in biomacromolecules for biomedical applications and generate bioactive surfaces to control biological systems.
|
News
|
17.06.2025 - ERC Advanced Grant
.jpg) |
Prof. Dr. Hans Börner wurde für sein Projekt IDefix mit einem ERC Advance Grant in Höhe von 2,5 Millionen geehrt und kann nun mit seinem interdisziplinären Team „Tiefe Sequenzdaten-analyse elektrochemisch schaltbarer Peptide nutzen, um Wege zu neuartigen Polymerkleb- und Funktionsstoffen“ zu erforschen. |
|
26.05.2025 - Congratulations to Dr. Steffen Busche!

We warmly congratulate Steffen Busche on successfully defending his PhD thesis "A Modular System for Personalized Support Materials by Inverse Electron Demand Diels-Alder Reaction of Norbornenes and 1,2,4,5-Tetrazines". A great achievement—congratulations, Dr. Busche!
|
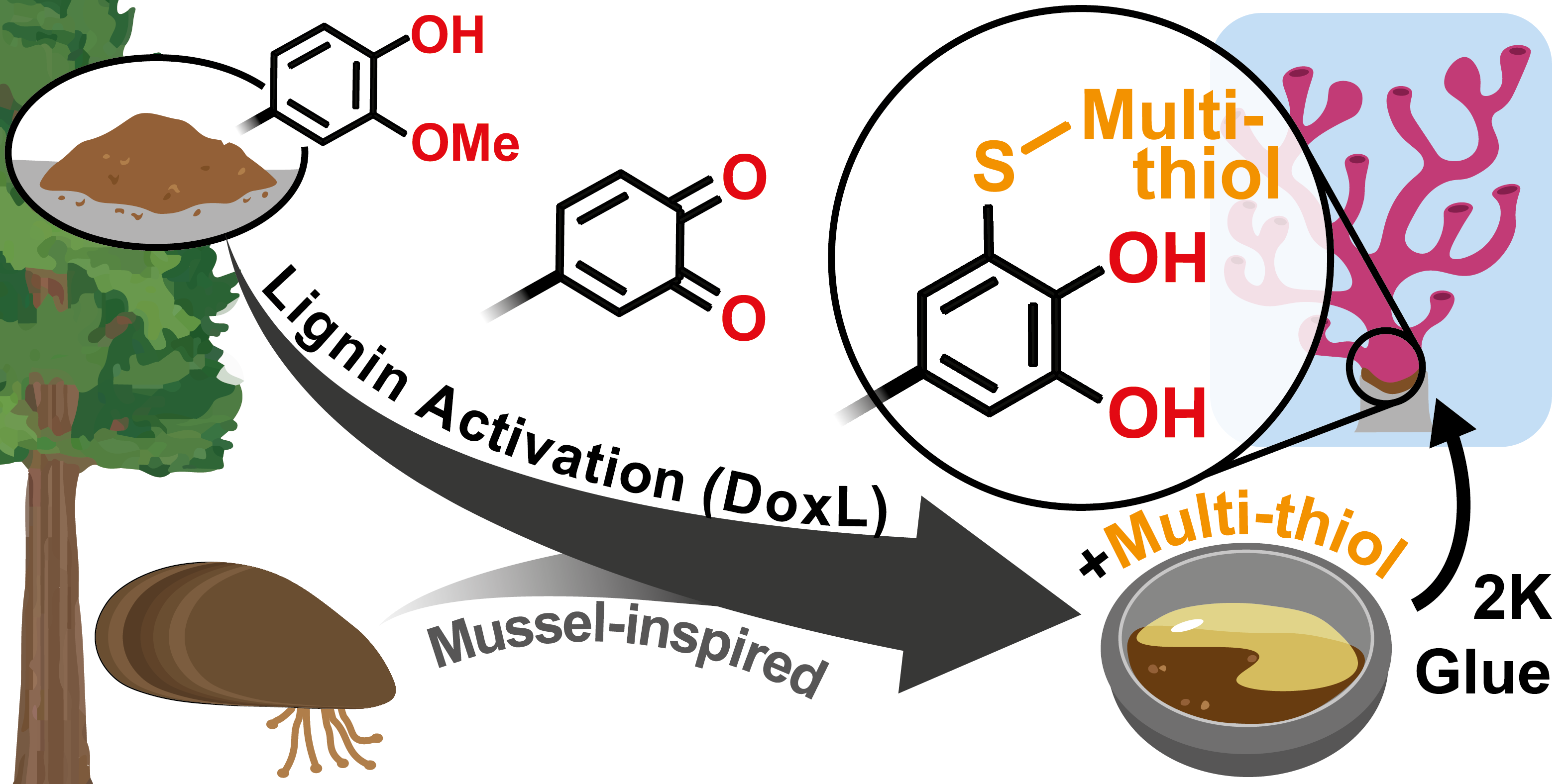
15.01.2025 - Electrosynthesis of mussel-inspired adhesive polymers as a novel class of transient enzyme stabilizers
New paper published in Angewandte Chemie by the electraDetouch team.
The electrochemical oxidation of multifunctional catechols represents a “green” and clean alternative pathway to ortho-quinones, which are used to form oligomer networks with branched multi-thiols. In these networks, the catechols are restored and represent adhesive points, which can establish transient interactions with enzymes. These oligomer/enzyme-conjugates are shielding the enzymes from heat stress, thus preventing denaturation. (https://doi.org/10.1002/anie.202419684)
|
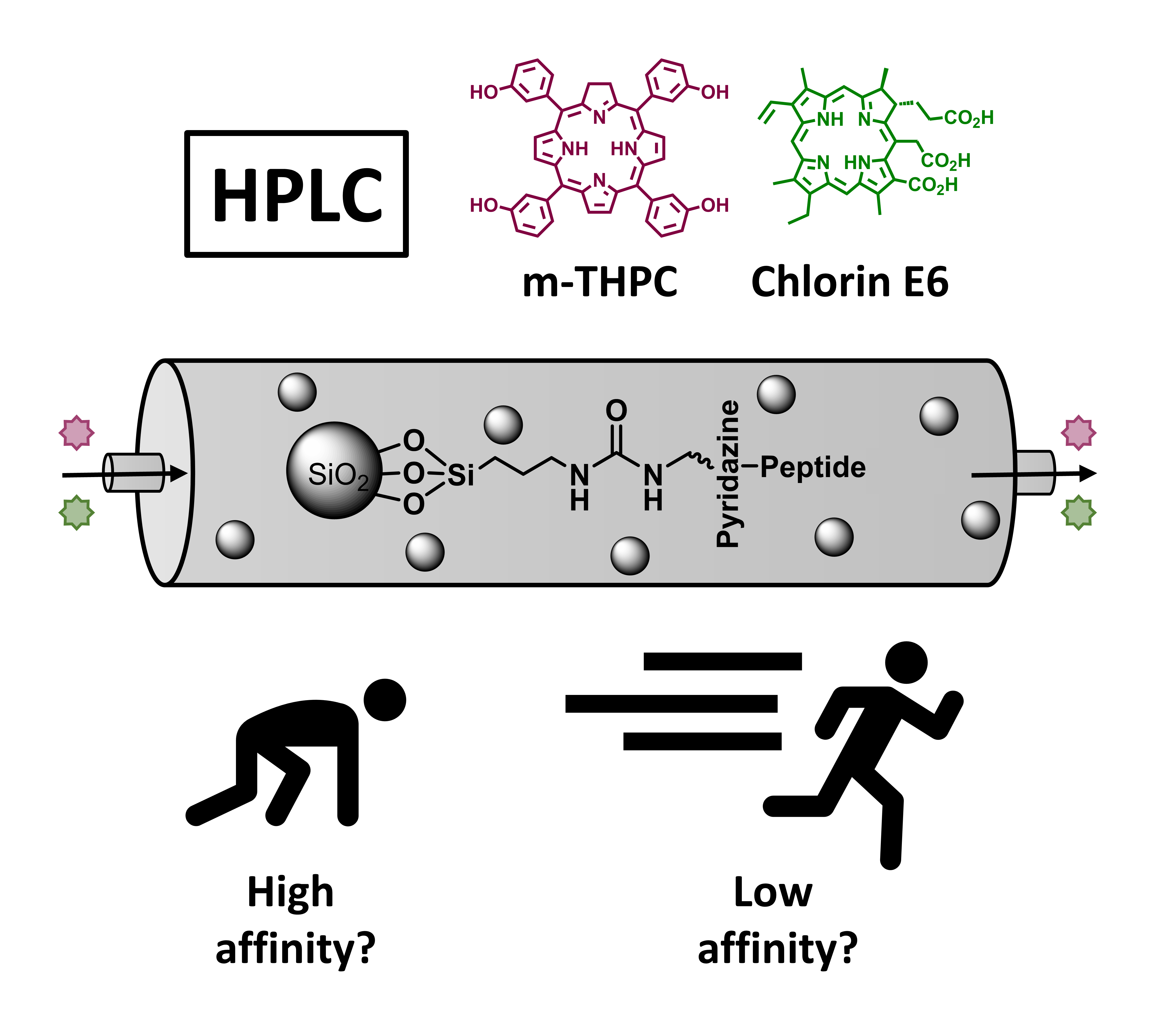
06.01.2025 - Toward Personalized Stationary Chromatography Phases: Equipping Commercial Silica Phases with Selected Peptide-Based Binding Domains to Tailor Affinity
The last paper of Steffen Busche was released in Advanced Materials Interfaces.
The 7-mer peptide motifs QFFLFFQ, QFFEFFQ and QFQQSFF, known as good, medium and poor binders for the photosensitizers m-THPC and Chlorin E6 are covalently immobilized on commercial amino-functionalized silica particles by inverse electron-demand Diels-Alder ligation. After proof of compound binding by UV/Vis batch experiments, professionally packed HPLC columns show compound specific separation performance with clear differences in retention times.
|
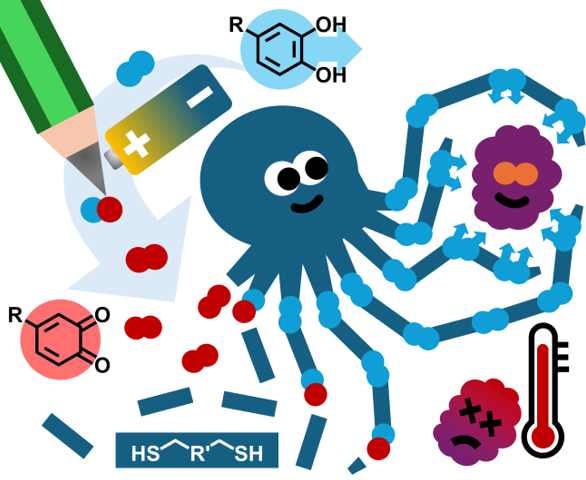
27.07.2024 - Redox-Triggered Debonding of Mussel-Inspired Pressure Sensitive Adhesives: Improving Efficiency Through Functional Design
New paper released in Angewandte Chemie.
Biomimetic mussel-inspired polymers based on thiol-catechol-connectivities are promising candidates for adhesives in a circular economy. However, the amount of simplification of the chemical structure can have an impact on the function of the polymer. Here, debonding by oxidation of TCC-catechols is demonstrated, comparing a fully synthetic monomer with a DOPA-based dipeptide. The dipeptide enhanced the debonding efficiency, combining smart design and natural feedstock – two critical aspects of circularity.
Full article link:
https://onlinelibrary.wiley.com/doi/10.1002/anie.202408441
https://onlinelibrary.wiley.com/doi/full/10.1002/ange.202408441
|
29.03.2024 - Enhancing Adhesion Properties of Commodity Polymers through Thiol-Catechol Connectivities: A Case Study on Polymerizing Polystyrene-Telechelics via Thiol-Quinone Michael-Polyaddition
New paper released in ACS Macro Letters.
Background: By incorporating only 3 mol% of thiol-catechol connectivities (TCCs) as functional adhesive groups into large polystyrene block copolymers could increase adhesive strength by up to 600% while keeping the integrity of the polymer segments.
Full article link:
https://doi.org/10.1021/acsmacrolett.4c00069
|
13.12.2023 - Organic transformation of lignin into mussel-inspired glues: next-generation 2K adhesive for setting corals under saltwater
New Paper released in Green Chemistry.
Background: The activation of lignin crosslinked with multi-thiol via thiol-catechol-connectivity (TCC) has led to the development of mussel-inspired and high-performing 2K adhesives that are ideal for setting corals in saltwater environments.
Full article link: https://doi.org/10.1039/D3GC03680D
|
17.11.2023 - Dissertation Award Berlin 2022 for Dr. Jana Krüger
Congratulations to our alumna Dr. Jana Krüger!
Background: The Dissertation Award Berlin, valued at 5000 €, is given out by Freie Universität Berlin, Humboldt-Universität zu Berlin and Technische Universität Berlin. Laureate of the 2022 award is Dr. Jana Krüger of Humboldt-Universität zu Berlin. She is being honored for her dissertation titled " Muschelinspirierte Polymerisation: Über die vollsynthetische Variante der enzymaktivierten Herstellung universeller Haftstoffe", written in the Börner-Group, which led to a new chemical platform for underwater adhesives.
Follow the link for the german version.
|
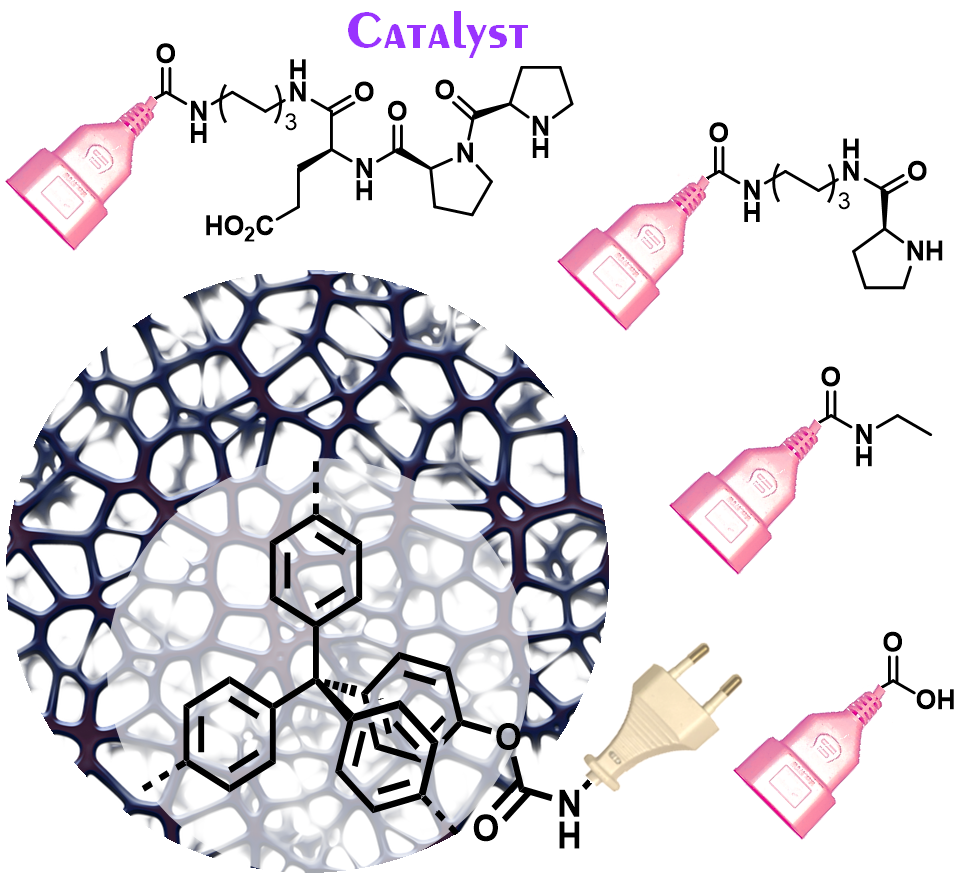
09.11.2023 - Ligating Catalytically Active Peptides onto Microporous Polymers: A General Route Toward Specifically-Functional High Surface Area Platforms
New Paper released in ChemSusChem.
Background: The bio-orthogonal tetrazine-norbornene ligation via an inverse electron-demand Diels-Alder reaction offers a generic route to the post-synthetic modification of microporous polymers. Equipping the porous scaffolds with norbornene derivatives enables click-like ligation to introduce functional tetrazines, including a peptide-based organocatalyst. This demonstrates the accessibility of the catalytic sites in the porous materials by achieving high activity and selectivity in an enamine catalysis.
Full article link: https://doi.org/10.1002/cssc.202301045
|
27.10.2023 - Two talk-prizes for our postdoc Dr. Tilmann Neubert
Congratulations to our postdoc Dr. Tilmann Neubert!
Tilmann was awarded with talk prizes at two conferences, the “2023 SALSA Make & Measure: Interfaces” (1st of 3 prizes, voted by the audience) and the "Sorrento 2013 – Sorrento 2023: A Decade of Peptide Materials” (best contributed oral presentation, awarded by the organizers).

Photos by Daniel Pasche (left) and Hans Börner (right)
|
| show all news |
|
|




.jpg)

.png)
.jpeg)