26.02.2026 - Gratulation an Keven Walter zum Promotionsabschluss mit summa cum laude!

Gratulation Keven Walter zum Abschluss der Promoten mit höchster Auszeichnung summa cum laude. Phantastische Resultate von neuen Monomeren bis zum Anwendungsdemonstrator. Debonding on command gets green and reality
|
06.01.2026 - Advancing Quantitative 31P NMR Spectroscopy for Reliable Thiol Group Analysis
New paper released in ACS Macro Letters
A straightforward 31P NMR spectroscopy protocol based on TMDP phosphitylation enables reliable thiol quantification in both small molecules and polymers. At the same time, the method allows the detection of hydroxy and carboxylate by-products, providing a rapid and comprehensive assessment of thiol-based (macro)molecules and their functional group composition.
DOI: https://pubs.acs.org/doi/10.1021/acsmacrolett.5c00739
|
21.11.2025 - Gratulation an Lukas Bangert zum erfolgreichen Promotionsabschluss!

Magna cum laude
Toller Endspurt und starke Methode für Papierkomposite!
|
31.10.2025 - New paper released in Polymer Chemistry!
Aromatic phenolic dithiols open new possibilities for bioinspired adhesives, reacting fast and cleanly with bisquinone A and producing polymers that maintain strong bonds even underwater, offering a path toward next-generation performance adhesives inspired by nature.
Full article link: https://doi.org/10.1039/D5PY00709G
|
31.10.2025 - New Paper Alert! TCC Polymers for Non-Covalent Paper Reinforcement
The adhesiveness and rigidity of aromatic TCC-polymers have been harnessed in the fabrication of purely non-covalent paper-polymer-composites. Using an in-sheet polymerization & adhesion (InSPA) approach, paper fibers could be homogeneously infused with hydrophobic polymers, resulting in significant mechanical reinforcement and water-repellent behavior. In contrast to commercial reinforcement agents, the coating could be fully extracted in a simple aqueous washing procedure, allowing for straightforward recycling of the underlying cellulose fibers.
DOI: https://doi.org/10.1021/acsami.5c13781
|
21.10.2025 - Honored Among Berlin’s Top 100 Scientists
I am honored to be included in Der Tagesspiegel's selection of "Die 100 wichtigsten Köpfe der Berliner Wissenschaft."
This recognition reflects not only individual research accomplishments but also the collective excellence of our research group, @BörnerLab @HU Berlin, whose work is driven by curiosity, scientific collaboration, and societal impact.
Both Berlin and Humboldt-Universität zu Berlin continue to stand as powerhouses of innovation, and it is a privilege to contribute to this research environment by developing advanced materials concepts for future technological applications.
|
17.06.2025 - ERC Advanced Grant
.jpg) |
Prof. Dr. Hans Börner wurde für sein Projekt IDefix mit einem ERC Advance Grant in Höhe von 2,5 Millionen geehrt und kann nun mit seinem interdisziplinären Team „Tiefe Sequenzdaten-analyse elektrochemisch schaltbarer Peptide nutzen, um Wege zu neuartigen Polymerkleb- und Funktionsstoffen“ zu erforschen. |
|
26.05.2025 - Congratulations to Dr. Steffen Busche!

We warmly congratulate Steffen Busche on successfully defending his PhD thesis "A Modular System for Personalized Support Materials by Inverse Electron Demand Diels-Alder Reaction of Norbornenes and 1,2,4,5-Tetrazines". A great achievement—congratulations, Dr. Busche!
|
15.01.2025 - Electrosynthesis of mussel-inspired adhesive polymers as a novel class of transient enzyme stabilizers
New paper published in Angewandte Chemie by the electraDetouch team.
The electrochemical oxidation of multifunctional catechols represents a “green” and clean alternative pathway to ortho-quinones, which are used to form oligomer networks with branched multi-thiols. In these networks, the catechols are restored and represent adhesive points, which can establish transient interactions with enzymes. These oligomer/enzyme-conjugates are shielding the enzymes from heat stress, thus preventing denaturation. (https://doi.org/10.1002/anie.202419684)
|
06.01.2025 - Toward Personalized Stationary Chromatography Phases: Equipping Commercial Silica Phases with Selected Peptide-Based Binding Domains to Tailor Affinity
The last paper of Steffen Busche was released in Advanced Materials Interfaces.
The 7-mer peptide motifs QFFLFFQ, QFFEFFQ and QFQQSFF, known as good, medium and poor binders for the photosensitizers m-THPC and Chlorin E6 are covalently immobilized on commercial amino-functionalized silica particles by inverse electron-demand Diels-Alder ligation. After proof of compound binding by UV/Vis batch experiments, professionally packed HPLC columns show compound specific separation performance with clear differences in retention times.
|
27.07.2024 - Redox-Triggered Debonding of Mussel-Inspired Pressure Sensitive Adhesives: Improving Efficiency Through Functional Design
New paper released in Angewandte Chemie.
Biomimetic mussel-inspired polymers based on thiol-catechol-connectivities are promising candidates for adhesives in a circular economy. However, the amount of simplification of the chemical structure can have an impact on the function of the polymer. Here, debonding by oxidation of TCC-catechols is demonstrated, comparing a fully synthetic monomer with a DOPA-based dipeptide. The dipeptide enhanced the debonding efficiency, combining smart design and natural feedstock – two critical aspects of circularity.
Full article link:
https://onlinelibrary.wiley.com/doi/10.1002/anie.202408441
https://onlinelibrary.wiley.com/doi/full/10.1002/ange.202408441
|
29.03.2024 - Enhancing Adhesion Properties of Commodity Polymers through Thiol-Catechol Connectivities: A Case Study on Polymerizing Polystyrene-Telechelics via Thiol-Quinone Michael-Polyaddition
New paper released in ACS Macro Letters.
Background: By incorporating only 3 mol% of thiol-catechol connectivities (TCCs) as functional adhesive groups into large polystyrene block copolymers could increase adhesive strength by up to 600% while keeping the integrity of the polymer segments.
Full article link:
https://doi.org/10.1021/acsmacrolett.4c00069
|
13.12.2023 - Organic transformation of lignin into mussel-inspired glues: next-generation 2K adhesive for setting corals under saltwater
New Paper released in Green Chemistry.
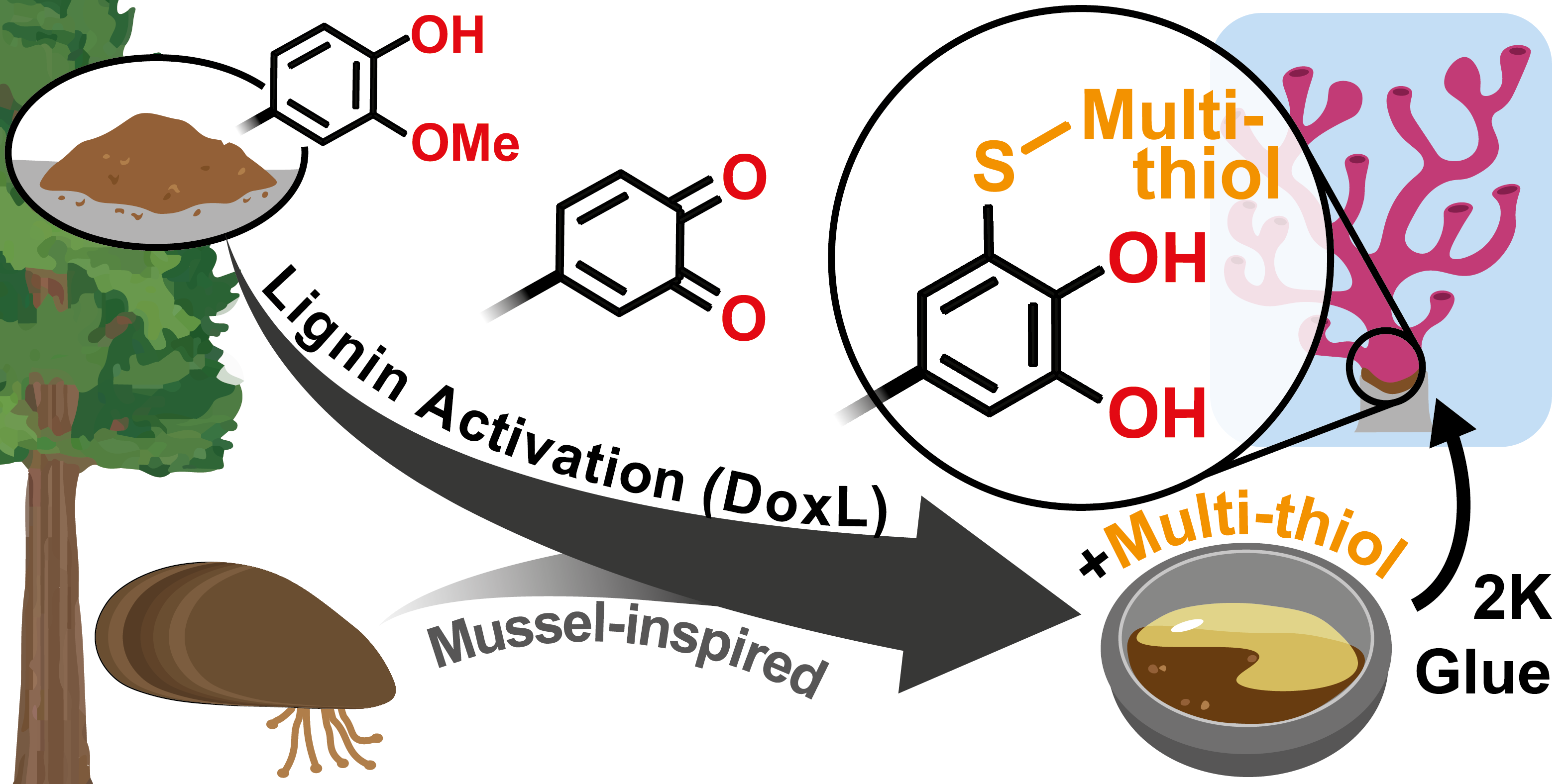
Background: The activation of lignin crosslinked with multi-thiol via thiol-catechol-connectivity (TCC) has led to the development of mussel-inspired and high-performing 2K adhesives that are ideal for setting corals in saltwater environments.
Full article link: https://doi.org/10.1039/D3GC03680D
|
17.11.2023 - Dissertation Award Berlin 2022 for Dr. Jana Krüger
Congratulations to our alumna Dr. Jana Krüger!
Background: The Dissertation Award Berlin, valued at 5000 €, is given out by Freie Universität Berlin, Humboldt-Universität zu Berlin and Technische Universität Berlin. Laureate of the 2022 award is Dr. Jana Krüger of Humboldt-Universität zu Berlin. She is being honored for her dissertation titled " Muschelinspirierte Polymerisation: Über die vollsynthetische Variante der enzymaktivierten Herstellung universeller Haftstoffe", written in the Börner-Group, which led to a new chemical platform for underwater adhesives.
Follow the link for the german version.
|
09.11.2023 - Ligating Catalytically Active Peptides onto Microporous Polymers: A General Route Toward Specifically-Functional High Surface Area Platforms
New Paper released in ChemSusChem.
Background: The bio-orthogonal tetrazine-norbornene ligation via an inverse electron-demand Diels-Alder reaction offers a generic route to the post-synthetic modification of microporous polymers. Equipping the porous scaffolds with norbornene derivatives enables click-like ligation to introduce functional tetrazines, including a peptide-based organocatalyst. This demonstrates the accessibility of the catalytic sites in the porous materials by achieving high activity and selectivity in an enamine catalysis.
Full article link: https://doi.org/10.1002/cssc.202301045
|
27.10.2023 - Two talk-prizes for our postdoc Dr. Tilmann Neubert
Congratulations to our postdoc Dr. Tilmann Neubert!
Tilmann was awarded with talk prizes at two conferences, the “2023 SALSA Make & Measure: Interfaces” (1st of 3 prizes, voted by the audience) and the "Sorrento 2013 – Sorrento 2023: A Decade of Peptide Materials” (best contributed oral presentation, awarded by the organizers).

Photos by Daniel Pasche (left) and Hans Börner (right)
|
01.09.2023 - Statistical Copolymers that Mimic Aspects of Mussel Adhesive Proteins: Access to Robust Adhesive-Domains for Non-Covalent Surface PEGylation
New Paper released in Macromolecular Rapid Communications.
Background: The robust anchoring of mussels to various substrates inspired the synthesis of a set of PEG- block-copolymers. Relevant side-chain functionalities of Dopa, Lys, and Arg residues found in mussel foot proteins are mimicked by functional acrylates to compose π-cation motifs. PEG- b-copolymers are synthesized by controlled radical polymerization. Waterborne coatings, show clear optima in stability and antifouling properties, preventing A549 cell adhesion.
Full article link: https://onlinelibrary.wiley.com/doi/10.1002/marc.202300300
|
07.08.2023 - Peptide-based Organocatalyst on Stage: Functionalizing Mesoporous Silica by Tetrazine-Norbornene Ligation
New Paper released in ChemCatChem
Background: Tetrazine-Norbornene ligation through inverse electron-demand Diels-Alder reaction has been employed as a novel strategy to immobilize a peptide-based catalyst onto different mesoporous silica supports. Functionalized silica monoliths as well as silica particles in packed bed reactors have been applied in the enantioselective flow catalysis of the addition reaction between ß-nitrostyrene and n-butanal.
Full article link: https://doi.org/10.1002/cctc.202300778
|
26.05.2023 - Tetrazine-norbornene versus azide-norbornene ligation: evaluating the toolbox for polymer–polymer coupling
New Paper released in Polymer Chemistry.
Background: Two segment-segment coupling strategies for accessing block-copolmyers are compared, investigating the ligation chemistries of norbornenes with either asymmetric tetratines or azides.
Full article link: https://doi.org/10.1039/D3PY00320E
|
17.09.2022 - Antimicrobial finish of polyethersulfone-membranes: Sticking photosensitizers like marine mussels would do
New Paper released in Advanced Engineering Materials.
A chlorin-based photosensitizer, known from photodynamic therapy was derivatized to realize antimicrobial finishes for water-filtration membranes. The introduced catechol moieties known from adhesive systems of marine mussels, improved the coating stability and affect both positioning and packing of the photosensitizers, to retain singlet oxygen production. The irradiation of coated membranes by visible light significantly reduces bacterial growth of both gram-positive and gram-negative strains.
Full article link: https://doi.org/10.1002/adem.202201279
|
30.05.2022 - Congratulations to Jana Krüger
Today Jana Krüger has defended her outstanding PhD theses „Muschelinspirierte Polymerisation: Über die vollsynthetische Variante der enzymaktivierten Herstellung universeller Haftstoffe“ with “summa cum laude”.
Congratulations and good luck for your future career!
|
25.01.2022 - Broadening the chemical space of mussel-inspired polymerization to roll out the TCC-polymer platform
New Paper released in Macromolecules.
Abstract: The mussel-inspired polymerization (MIPoly) of bisquinone (AA type) and dithiol (BB type) monomers utilizes room temperature Michael-type polyaddition to form polymers with adhesive thiol–catechol connectivities (TCCs) in their backbone. The combination of five bisquinones and eight dithiols proves the generic character of this robust polymerization and leads to a TCC-polymer library with 40 different polymers. The set of adhesives is investigated in detail, and structure–property relationships are studied, analyzing material properties and adhesive capabilities. Dry adhesive tests are carried out under hot-melt-like conditions, revealing adhesive strengths up to 2.40 MPa for gluing aluminum and 1.26 MPa for polypropylene. A selected set of TCC adhesives is further studied under seawater-model conditions for the wet-gluing and wet-curing of technical aluminum substrates. The library approach offers access to novel adhesives in the field of mussel-inspired glues as shown by seawater-tolerant adhesives that are providing adhesive strengths of up to 1.25 MPa under hostile high-salt conditions.
Full article link: https://doi.org/10.1021/acs.macromol.1c02192
|
01.12.2021 - BMBF-Projekt
In der BMBF Förderlinie „Zukunftstechnologien für die industrielle Bioökonomie: Schwerpunkt Biohybride Technologien“ wurde das Verbundprojekt unter der Koordination der HU Berlin erfolgreich eingeworben. Zu dem Thema „Biohybride Klebsysteme: Über enzymaktivierte Polymerisation zur Technologieplattform der Adhäsionsausschaltung auf Knopfdruck“ forscht der Verbund aus HU, Henkel, Fraunhofer IAP und INURU an neuen, schaltbaren Klebstoffen die für zukünftige Rezyklierungsstrategien in einer modernen Kreislaufwirtschaft dringlich notwendig sind.
|
11.08.2021 - BMBF-Projekt
Im BMBF Ideenwettbewerb „Biologisierung der Technik“ wurde das Projekt „LigNovolac“ erfolgreich eingeworben. Unter dem Thema „Kleben wie Holz und Muscheln: Nachhaltige Klebstoffe für Korallenriff-Rekonstitution“ forscht ein Team aus dem AK Börner in Zusammenarbeit mit Henkel an neuen Klebstoffen, die zur Setzung von temperaturresistenten Korallen für die Rettung geschädigter Korallenriffe dringlich notwendig sind.
Abb. 1.: Unterwasserkleber können maßgeschneiderte Lösungen aus biologischen Klebesystemen mit Nachhaltigkeit und ökologischer Verträglichkeit verbinden.
|
01.04.2021 - Jana Maria Krüger was awarded with a Talk Prize at 23rd JCF-Frühjahrssymposium
Congratulations to Jana Maria Krüger! At the 23rd JCF-Frühjahrssymposium her presentation titled "Paving the way toward a material platform via mussel-inspired polymerization" was awarded with a Talk Prize (Second Place). The 23rd JCF-Frühjahrssymposium was organized by JCF from Leipzig, Halle/Saale, Dresden and Berlin and took place in the online venue Gather.Town.
|
18.02.2021 - Congratulations to Narendra Lagumaddepalli Venkatareddy
Today Narendra Lagumaddepalli Venkatareddy has defended his outstanding PhD theses „Revealing secrets of mussel-glue mimetic peptides; From advanced NMR analysis to computational process modellings“.
Congratulations and good luck for your future career!
|
05.02.2021 - Implementing Zn2+ ion and pH-value control into artificial mussel glue proteins by abstracting a His-rich domain form preCollagen
New Paper released in Soft Matter.
Abstract: A His-rich domain of preCollagen-D found in byssal threads is derivatized with Cys and Dopa flanks to allow for mussel-inspired polymerization. Artificial mussel glue proteins are accessed that combine cysteinyldopa for adhesion with sequences for pH or Zn2+induced β-sheet formation. The artificial constructs show strong adsorption to Al2O3, the resulting coatings tolerate hypersaline conditions and cohesion is improved by activating the β-sheet formation, that enhances E-modulus up to 60%.
Full article link: https://doi.org/10.1039/D0SM02118K
|
28.01.2021 - Accessing the Next Generation of Synthetic Mussel-Glue Polymers via Mussel-Inspired Polymerization
New Paper released in Angewandte Chemie International Edition.
Abstract: The formation of cysteinyldopa as biogenic connectivity in proteins is used to inspire a chemical pathway toward mussel-adhesive mimics. The mussel-inspired polymerization (MIPoly) exploits the chemically diverse family of bisphenol monomers that is oxidizable with 2-iodoxybenzoic acid to give bisquinones. Those react at room temperature with dithiols in Michael-type polyaddition, leading to polymers with thiol–catechol–connectivities (TCC). A set of TCC-polymers prove adhesive behavior even on challenging poly(propylene) substrates and there they compete in dry adhesive strength with commercial epoxy resins. The MIPoly promises ease of scale up and exhibits high modularity to tailor adhesives, as proven on a small library, where one candidate shows wet adhesion on aluminum substrates in both water and sea water models.
Full article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202015833
|
24.11.2020 - Information-Based Design of Polymeric Drug Formulation Additives
New Paper released in Biomacromolecules 2020.
Abstract: Tailor-made copolymers are designed based on a peptide-poly(ethylene glycol) (QFFLFFQ-PEG) conjugate as a blueprint, to solubilize the photosensitizer meta-tetra(hydroxyphenyl)chlorin (m-THPC). The relevant functionalities of the parent peptide-PEG are mimicked by employing monomer pairs that copolymerize in a strictly alternating manner. While styrene (S) or 4-vinylbenzyl-phthalimide (VBP) provide aromatic moieties like Phe, the aliphatic isobutyl side chain of Leu4 is mimicked by maleic anhydride (MA) that reacts after polymerization with isobutylamine to give the isobutylamide-carboxyl functional unit (iBuMA). A set of copolymer-PEG solubilizers is synthesized by controlled radical polymerization, systematically altering the length of the functional segment (DPn = 2, 4, 6) and the side chain functionalization (iBuMA, iPrMA, MeMA). The m-THPC hosting and release properties of P[S-alt-iBuMA]6-PEG reached higher payload capacities and more favored release rates than the parent peptide-PEG conjugate. Interestingly, P[S-alt-RMA]n-PEG mimics the sensitivity of the peptide-PEG solubilizer well, where the exchange of Leu4 residue by Val and Ala significantly reduces the drug loading by 92%. A similar trend is found with P[S-alt-RMA]n-PEG as the exchange of iBu → iPr → Me reduces the payload capacity up to 78%.
|
27.08.2020 - Combining Phage Display and Next-Generation Sequencing for Materials Sciences: A Case Study on Probing Polypropylene Surfaces
New Paper released in Journal of the American Chemical Society.
Phage display biopanning with Illumina next-generation sequencing (NGS) is applied to reveal insights into peptide-based adhesion domains for polypropylene (PP). One biopanning round followed by NGS selects robust PP-binding peptides that are not evident by Sanger sequencing. NGS provides a significant statistical base that enables motif analysis, statistics on positional residue depletion/enrichment, and data analysis to suppress false-positive sequences from amplification bias. The selected sequences are employed as water-based primers for PP–metal adhesion to condition PP surfaces and increase adhesive strength by 100% relative to nonprimed PP.
Full article link: https://pubs.acs.org/doi/full/10.1021/jacs.0c03482
|
21.08.2020 - Congratulations to Carmen Juds
Today Carmen Juds has defended her outstanding PhD theses „Phagendisplay und Hochdurchsatz- Sequenzierung: Neue Werkzeuge zur Identifizierung Peptid-basierter Materialbinder“.
Congratulations and good luck for your future career!
|
28.07.2020 - Toward Artificial Mussel-Glue Proteins: Differentiating Sequence Modules for Adhesion and Switchable Cohesion
New Paper released in Angewandte Chemie International Edition.
Abstract: Artificial mussel-glue proteins with pH-triggered cohesion control were synthesized by extending the tyrosinase activated polymerization of peptides to sequences with specific modules for cohesion control. The high propensity of these sequence sections to adopt b-sheets is suppressed by switch defects. This allows enzymatic activation and polymerization to proceed undisturbed. The b-sheet formation is regained after polymerization by changing the pH from 5.5 to 6.8, thereby triggering O→N acyl transfer rearrangements that activate the cohesion mechanism. The resulting artificial mussel glue proteins exhibit rapid adsorption on alumina surfaces. The coatings resist harsh hypersaline conditions, and reach remarkable adhesive energies of 2.64 mJm-2 on silica at pH 6.8. In in situ switch experiments, the minor pH change increases the adhesive properties of a coating by 300% and nanoindentation confirms the cohesion mechanism to improve bulk stiffness by around 200%.
Full article link: https://doi.org/10.1002/ange.202008515
|
17.06.2020 - Congratulations to Emmanuelle Schue
Today Emmanuelle Schue has defended her outstanding PhD theses „Oxidative intramolecular crosslinking in sequence-controlled polymers: Approaches toward more complex designs and folding analysis “.
Congratulations and good luck for your future career!
|
03.06.2020 - Congratulations to Matthias Röber!
Matthias Röber absolvierte die erste digitale Promotionsverteidigung im AK Börner und schloss damit seine Promotion zum Thema „Templatgeleitete Strukturbildung kollagenartiger Peptide" erfolgreich ab.
Wir gratulieren zum Doktortitel und zur Einstellung bei Pensatech Pharma GmbH.
|
18.02.2020 - Student internship in polymer chemistry
Today we welcomed students of the Gorge-Orwell-school from Berlin Lichtenberg for a laboratory internship in polymer chemistry.
|
27.11.2019 - Congratulations to Justus Horsch!
Today Justus Horsch has defended his outstanding PhD theses „Muschel-inspirierte Polymerisation: Synthetische Bioadhäsive für wasserbasierte Klebstoffe und meerwasserresistente Beschichtungen“ with “summa cum laude”.
Congratulations and good luck for your future career!
|
06.11.2019 - Mussel‐Inspired Polymerization of Peptides: The Chemical Activation Route as Key to Broaden the Sequential Space of Artificial Mussel‐Glue Proteins
New Paper released in Macromolecular Rapid Communications.
Background: A chemical activation route makes the use of tyrosinase in the mussel‐inspired polymerization of peptides obsolete and enables the exploitation of the sequence space to generate artificial mussel‐glue proteins. This is shown by polymerizing the minimal adsorption domain Dopa‐Lys‐Cys, leading to poly(Dopa‐Lys‐Cys) with non‐peptidic thiol–catechol connectivities in the backbone. Polymer films are formed under seawater conditions and resist washing with 4.2 M hypersaline solution.
Full article link: https://onlinelibrary.wiley.com/doi/full/10.1002/marc.201900431
|
22.07.2019 - Learning from peptides to access functional precision polymer sequences
New Paper in Angewandte Chemie has been published and is highlighted as hot article in drug delivery research:
Background: A new strategy to guide the sequence design for accessing functional precision polymers was described. For that purpose, a well-studied peptide that acts as a formulation additive to solubilize a photosensitizer drug was used and the direct translation of side chain functionality sequences towards precision polymer backbones, leads to macromolecules, which mimic drug hosting and release properties of the parent peptide.
Full article Link:
https://doi.org/10.1002/anie.201902217
Hot Topic: Drug Delivery:
https://onlinelibrary.wiley.com/doi/toc/10.1002/(ISSN)1860-7187.hottopic-drugdelivery
|
17.07.2019 - ILLUMINATING BIONANO INTERFACES
|
11.06.2019 - Fish and Clips: A Convenient Strategy to Identify Tyrosinase Substrates with Rapid Activation Behavior for Materials Science Applications
New paper released in ACS Macro Letters:
Background: A Method for the identification of peptides with suitable substrate properties for the enzyme tyrosinase was developed by labeling of enzymatically activated solid-phase supported peptides in a one-bead-one-compound library with a fluorescent probe. This was achieved by Michael addition of a thiol-functionalized probe to dopaquinone residues resulting from enzymatic tyrosine oxidation. Activation kinetics of identified peptides verified the selection process and coating experiments underlined the applicability for materials science.
doi: 10.1021/acsmacrolett.9b00244
|
04.06.2019 - A strategy to better understand biological adhesion processes
|
06.05.2019 - Mussel-Glue Inspired Adhesives: A Study on the Relevance of L-Dopa and the Function of the Sequence at Nanomaterial-Peptide Interfaces
New Paper in Advanced Material Interfaces has been published
Background: A mussel-glue inspired peptide obtained via phage display screening mimics aspects of mussel glue proteins by undergoing distinct structural responses at the nanomaterial interface to optimize binding kinetics and surface contacts. NMR in combination with molecular dynamics simulation provides molecular level insights into the structure of the surface- bound adhesive peptide and enables to suggest an adhesion process.
doi: 10.1002/admi.201900501
|
15.10.2018 - Kekulé Fellowship of the VCI
We congratulate Jana Kohn for receiving the Kekulé Fellowship of the VCI for two years. She will work on an enzyme-free route towards synthetic mussel-inspired polymers.
|
11.07.2018 - On the way to precision formulation additives: 2D-screening to select solubilizers with tailored host and release capabilities
New Paper in Journal of Controlled Release
Background: A 2-dimensional high-throughput screening method is presented to select peptide sequences from large peptide libraries for precision formulation additives, having a high capacity to specifically host a drug of interest and provide tailored drug release properties. The identified sequences are conjugated with poly(ethylene glycol) (PEG) to obtain peptide-PEG conjugates that proved to be valuable as solubilizers for small organic molecule drugs to overcome limitations of poor water-solubility and low bio-availability.
doi: 10.1016/j.jconrel.2018.06.032
|
01.04.2018 - Humboldt Fellowship for Postdoctoral Researchers
Dr. Sandra Arias has joined the group with an Alexander von Humboldt Fellowship for Postdoctoral Researchers for two years to work in Mussel-inspired adhesives by switchable β-sheet segments. Congratulations!
|
29.11.2017 - Engineered collagen - a redox switchable framework for tunable assembly and fabrication of biocompatible surfaces
New Paper in ACS Biomaterials Science & Engineering has been published.
Background: Collagen, processed into several morphologies and originating from various sources, has long since been used as a biocompatible material that can assist wound healing and tissue regeneration. With the advent of biotechnology and solidphase peptide synthesis, new possibilities arise to create rationally designed biomaterials based on collagen sequences incorporating new functionalities whilst maintaining the beneficial properties of natural collagen. In this study a new class of synthetic collagen materials is presented, defined by its simplistic core structure and its therefore predictable behavior.
doi: 10.1021/acsbiomaterials.7b00583
|
16.11.2017 - PEGylated Precision Segments Based on Sequence-Defined Thiolactone Oligomers
New Paper in Macromolecular Rapid Communications has been published.
Background: A straightforward access route to multifunctional block copolymers, combining a poly(ethylene glycol) (PEG) block and a monodisperse segment with discrete monomer sequence based on thiolactone chemistry, is described. Exploiting an inverse conjugation strategy on a PEG preloaded poly(styrene) synthesis resin enables the convenient introduction of a predefined PEG-block at the α-terminus of thiolactone-based sequence-defined oligomers.
doi: 10.1002/marc.201700688
|
07.11.2017 - Identification of Functional Peptide Sequences to Lead the Design of Precision Polymers
New Review paper in Macromolecular Rapid Communications has been published.
Background: Peptide sciences developed dramatically as a result of routine use of solidphase peptide synthesis and nowadays offer a rich set of well-established strategies to design and identify functional peptide sequences for advanced applications in materials sciences. Appropriate sequences for a wide range of interesting material targets, ranging from molecules to materials surfaces and internal interfaces, can be selected via combinatorial means, and sequence specificities within the resulting peptide–target interactions can be routinely investigated. Based on this understanding, macromolecular sciences can define new polymer structures that meet required functionalities or functional sequences with fully synthetic, nonpeptidic precision polymers to endeavor toward information-based design of next-generation, purpose-adapted macromolecules.
doi: 10.1002/marc.201700632
|
15.03.2017 - Fine-tuning Nanocarriers Specifically toward Cargo: A Competitive Study on Solubilizing Related Photosensitizers for Photodynamic Therapy
New Paper in Bioconjugate Chemistry has been published.
Background: Tailor-made drug solubilizers are studied based on peptide-poly(ethylene glycol) conjugates, which exhibit peptide segments constituting binding motifs for the small-molecule drugs of interest to render them water-soluble. Suitable 7mer peptides are selected via combinatorial means by screening large one-bead-one-compound (OBOC) peptide libraries. The capability of the screening method to read out structural detail of the drugs is investigated by comparing three related photosensitizers (Chlorin E6 (Ce6), Pheophorbide A (Pba) and meta-tetra(hydroxyphenyl)chlorin (m-THPC), which are applicable for photodynamic cancer therapy.
doi: 10.1021/acs.bioconjchem.6b00549
|
03.01.2017 - Inhibition of Tau protein aggregation by rhodanine-based compounds solubilized via specific formulation additives to improve bioavailability and cell viability
New Paper in Current Alzheimer Research has been published.
Background: Anti-aggregation drugs play an important role in therapeutic approaches for Alzheimer’s disease. We have previously developed a number of compounds that are able to inhibit the pathological aggregation of Tau protein. One common obstacle to application is the limited penetration across the plasma membranes into cells, where Tau aggregation occurs in the cytosol. We used an inducible N2a cell line which expresses the repeat domain of tau and develops tau aggregates.
|
12.12.2016 - Easy access to triazolinedione-endcapped peptides for chemical ligation
New Paper in Chemical Communications has been published
A triazolinedione-precursor is directly built up from N-terminal peptide amine on resin enabling a versatile route towards peptide-polymer conjugates.
doi: 10.1039/c6cc08683g
|
06.12.2016 - SALSA Poster Session
CJ was rewarded the poster award of the SALSA Poster Session 2016. Congratulations!
The annual poster session of the School of Analytical Sciences Adlershof took place at the chemistry institute (Campus Adlershof) on December 6th, 2016. In the course of this event, CJ won a poster award. Congratulations! ( link)
|
14.10.2016 - Chapter 1: Synthetic Aspects of Peptide- and Protein-Polymer Conjugates in the Post-click Era.
New book chapter in RSC Polymer Chemistry Series No. 22: Bio-inspired Polymers has been published.
This book chapter will provide an insight into the chemical synthesis of peptide- and protein-polymer conjugates, ranging from classical synthetic reactions, to efficient bioorthogonal strategies, novel bioconjugation techniques and chemoenzymatic approaches. Against the background of bio-inspiration, the book will give a comprehensive review of novel polymer synthesis, adaptive composites and bio-mimetic surfaces. Diverse applications of bio-inspired polymers in fields such as tissue engineering, drug delivery, optical materials and many more are described.
doi: 10.1039/9781782626664-00001
|
01.08.2016 - Advancing Drug Formulation Additives toward Precision Additives with Release Mediating Peptide Interlayer
New Paper in Journal of the American Chemical Society has been published
doi: 10.1021/jacs.6b03604
|
04.06.2016 - Easy Access toward Functional Patterns on Cellulose Paper by Combining Laser Printing and Material-Specific Peptide Adsorption
New Paper in Angewandte Chemie has been published
Stick to print: Selective coating of laser-printed patterns was achieved by material-specific adhesion of peptides to cellulose or toner. The peptide sequences were obtained using phage display. Functionalization of the immobilized peptides was realized by selective modification of tyrosine residues present on the printed-pattern coatings, offering new ways towards low-cost printed devices.
doi: 10.1002/anie.201601603
|
19.04.2016 - Enzyme-triggered antifouling coatings: switching bioconjugate adsorption via proteolytically cleavable interfering domains.
New paper in ACS Macro Letters has been published.
The enzymatic activation of non-adhesive peptide-polymer conjugates by proteoytic cleavage of suitable interfering domains results in stable surface coatings. An efficient reduction of protein/surface interactions is shown.
doi: 10.1021/acsmacrolett.6b00072
|
12.06.2015 - 4th International Conference
Felix Hanßke was rewarded a poster award. Congratulations!
From June 10 to 12, 2015 The 4th International Conference "Strategies in Tissue Engineering" of WITE e.V. took place at the Congress Center Würzburg. In the course of this event, Mr. Felix Hanßke won the poster award for the best “materials” poster. Congratulations! ( link)
|
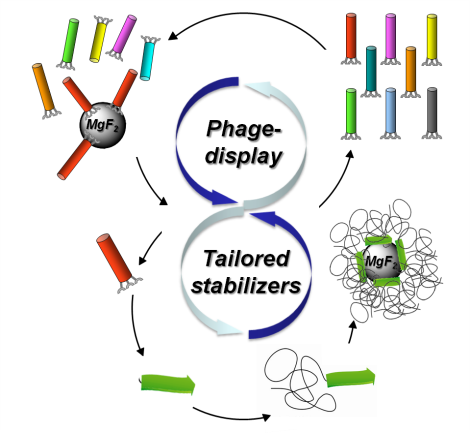
08.06.2015 - Generic biocombinatorial strategy to select tailor-made stabilizers for sol-gel nanoparticle synthesis.
|
New Paper in Small has been published.
A generic route toward de novo design of tailor-made stabilizers for sol-gel nanoparticle synthesis is described. By exploiting phage display biopanning to select stabilizers from large peptide libraries, a significantly larger chemical space (~ 109 different compounds) is explored, compared to commonly used knowledge-based or empiric strategies. The approach is demonstrated on the fluorolytic sol-gel synthesis of MgF2 nanoparticles from Mg(OCH3)2 precursors. Peptide sequences are selected, showing sequence-specific adsorption onto MgF2 particle surfaces.
doi: 10.1002/smll.201500162
|
 |
|
05.05.2015 - School of Analytical Sciences Adlershof (SALSA) Poster Session 2015
Valeria Samsoninkova was rewarded the SALSA “Best Design” Poster award 2015. Congratulations! ( link)
|
09.04.2015 - 4. Berliner Chemie Symposium (BCS)
Felix Hanßke was rewarded the poster award of the BCS/EYCN Meeting 2015. Congratulations!
The 4rd Berliner Chemie Symposium (BCS) together with the annual European Young Chemists Network (EYCN) meeting took place at the Erwin Schrödinger Zentrum (Campus Adlershof) on April 9th, 2015. In the course of this event, Mr. Felix Hanßke won a poster award. Congratulations! ( link)
|
19.03.2015 - Synthesis of conjugates combining macromolecular brushes and rigid macrocycles.
|
New Paper in Polymer has been published.
On the occasion of the 65th birthday of Krzysztof Matyjaszewski ( Link) a synthesis route to a conjugate comprised of two macromolecular brushes and a central shape persistent macrocycle utilizing ATRP is presented. As a result of the properties of the combined structure elements a functional macromolecule is obtained that regulates accessibility to a central shape persistent macrocycle via reversible brush-coil transitions.
doi:10.1016/j.polymer.2015.02.059 |
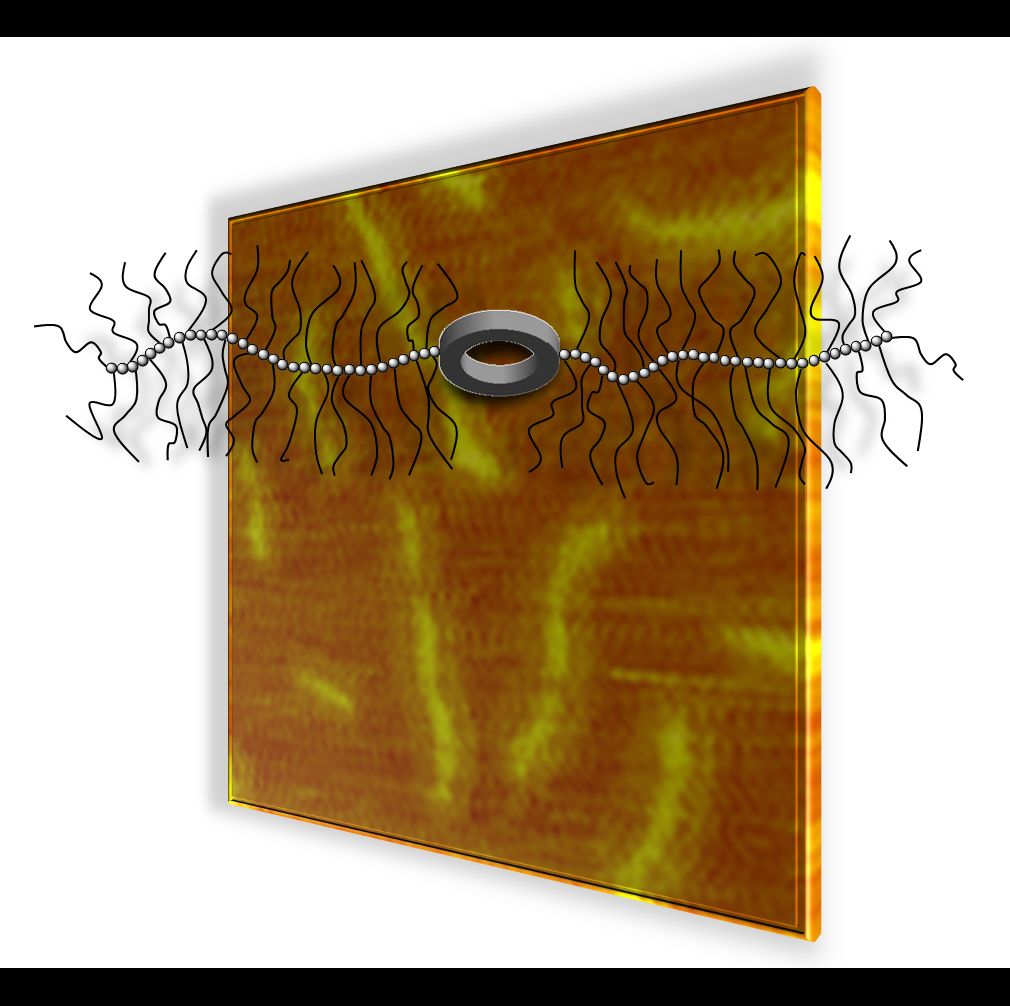 |
|
19.11.2014 - Forum Junge Spitzenforscher: New Materials - Award
Patrick Wilke received:
Innovation award 2014 from Humboldt Innovation in collaboration with Stiftung Industrieforschung for the presentation “Ein biokombinatorischer Ansatz zu aktivierbaren, muschelinspirierten Adhäsiven”.
|
01.09.2014 - A direct biocombinatorial strategy towards next generation, mussel-glue inspired saltwater adhesives.
|
New Paper in Journal of the American Chemical Society has been published.
Biological materials exhibit remarkable, purpose-adapted properties that provide source of inspiration for designing new materials to meet the requirements of future applications. For instance, marine mussels are able to attach to a broad spectrum of hard surfaces under hostile conditions. Controlling wet-adhesion of synthetic macromolecules by analogue processes promises to strongly impact materials sciences by offering advanced coatings, adhesives and glues.
|
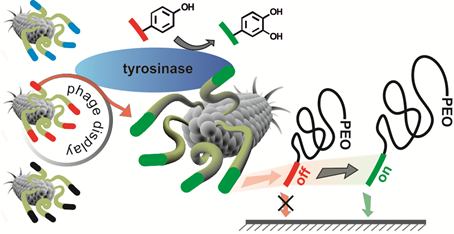 |
|
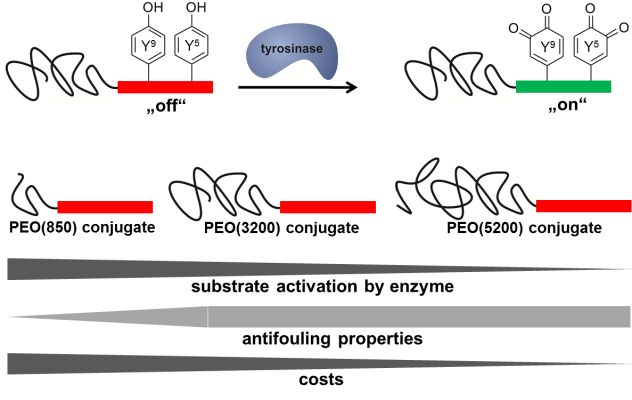
01.09.2014 - Revealing the impact of poly(ethylene oxide) blocks on enzyme activable coatings from peptide-polymer conjugates
|
New Paper in European Polymer Journal has been published.
Peptide-polymer conjugates composed of a poly(ethylene oxide) (PEO) block and a precursor segment from mussel foot protein-1 (mefp-1) are enzymatically oxidized by tyrosinase. A functional transition from weak/reversible binders to strong/irreversible adsorption onto aluminum oxide surfaces is observed. To elucidate effects of PEO-block length on the enzyme activable formation of antifouling coatings on aluminum oxide surfaces, a set of mefp-1-block-PEO bioconjugates with PEO-block lengths of 850, 3200 and 5200 g/mol is synthesized and investigated.
|
 |
|
28.08.2014 - Hans Börner and Stefan Hecht (Humboldt-Universität zu Berlin, Berlin) organized the 2nd ERC-Grantees Conference on „Frontiers in Chemistry - The Basis for Advanced Materials“
It is our great pleasure to invite you the 2nd European meeting of ERC grantees in chemistry to be held in Berlin on August 28 & 29, 2014. The meeting Frontiers of Chemistry continues a tradition started in Strasbourg two years ago.
The aim of the conference is to gather some of the leading European experts in the field of chemical materials research, showcase their latest achievements, and discuss about future trends.
Weblink
|
 |
|
25.05.2014 - J.-F. Lutz (Institut Charles Sadron, UPR22 CNRS, Strasbourg) and H. G. Börner (Humboldt-Universität zu Berlin, Berlin) organized the EUPOC 2014 on precision polymers.
The objective of this conference will be to discuss a new side of macromolecular research: “Precision Polymers”. In particular, this conference will highlight new directions in polymer synthesis. Indeed, we believe that the polymer chemists of the 21st century have to explore options, which are beyond conventional macromolecular engineering. For instance, the control over polymer architectures is nowadays not a challenge anymore. Recent tools such as controlled radical polymerizations or “click” chemistry allow a fine control of polymer architectures.
Weblink
|
15.05.2014 - Congratulations to Prof. Dr. Laura Hartmann
Congratulations to Laura Hartmann, who successfully defended her habilitation at the FU-Berlin and received a call to the W3 professor position at Heinrich Heine University of Düsseldorf (Nachfolge Ritter). We are very happy about the first academic offspring from the Börner Lab as Laura achieved her Ph.D. with us from 2004 to 2007.
Weblink
|
09.04.2014 - 3. Berliner Chemie Symposium (BCS)
Steffi Große was rewarded the poster award of the BCS 2014. Congratulations!
The 3rd Berliner Chemie Symposium (BCS) took place at the Erwin Schrödinger Zentrum (Campus Adlershof) on April 9th, 2014. In the course of this event, Ms. Steffi Große won a poster award. Congratulations! ( link)
|
05.12.2013 - Complex Single-chain Polymer Topologies locked by Positionable Twin Disulfide Cyclic Bridges
|
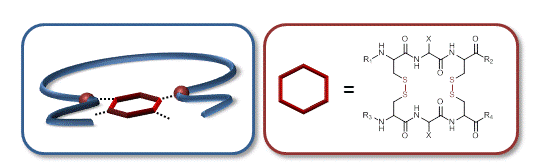
New Paper published in collaboration with Jean-François Lutz.
Oligomers containing the peptide sequence cysteine-anycysteine (CXC) were attached, at specific locations, to a linear chain of polystyrene. The polymer-bound peptide motifs were then oxidized in dilute conditions to afford a complex bio hybrid bi-cyclic topology via intramolecular twin disulfide bridge formation.
PDF |
 |
|
28.10.2013 - School of Analytical Sciences Adlershof
|
24.07.2013 - Spatially Controlled Surface Immobilization of Nonmodified Peptides
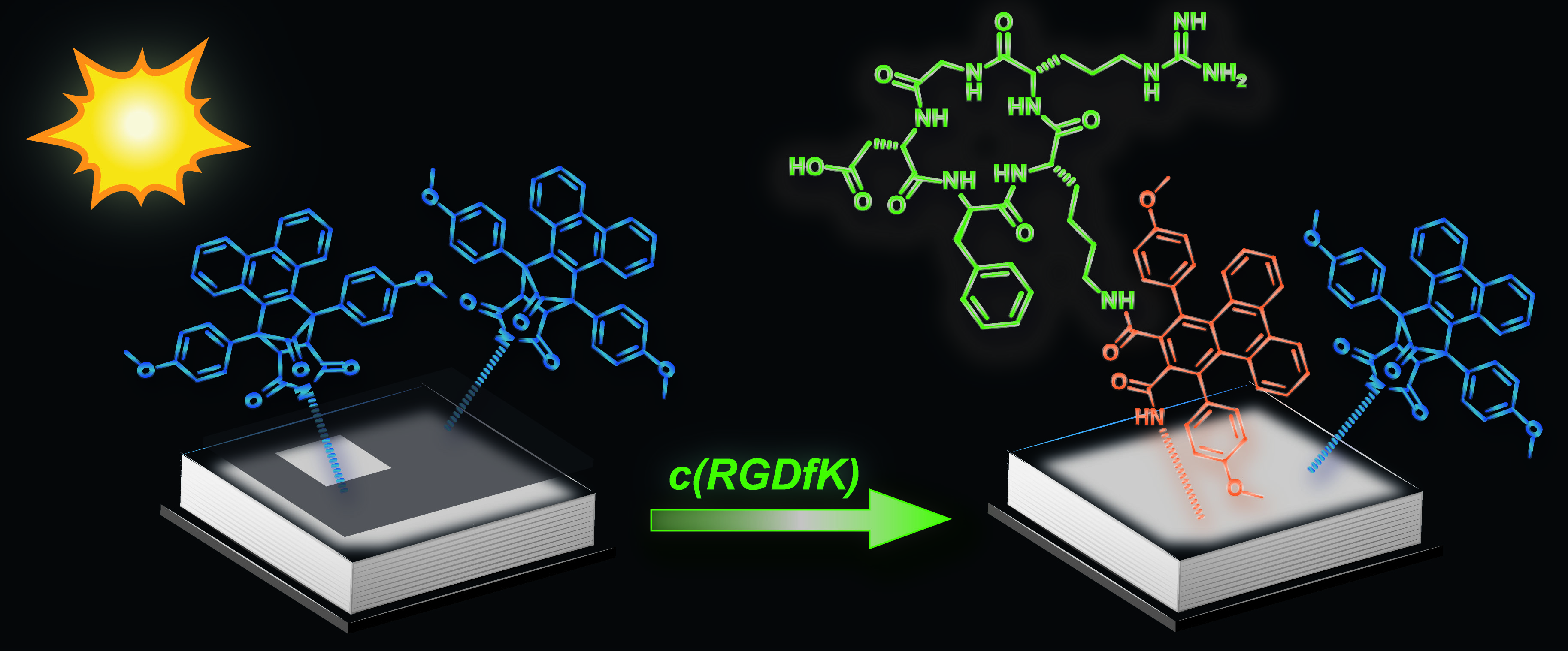
New Paper published in collaboration with Christopher Barner-Kowollik (KIT).
A phencyclone derivative is used to achieve light-controlled immobilization of peptides possessing only natural amino acids. The photoactive precursor (blue in picture) is formed in a Diels–Alder reaction and can undergo light-triggered ring-opening reactions with amines. Successful surface patterning with a genuine c(RGDfK) peptide (green) is evidenced by imaging time-of-flight secondary-ion mass spectrometry (ToF-SIMS).
PDF |
 |
|
12.07.2013 - Convenient Routes to Efficiently N-PEGylated Peptides
|
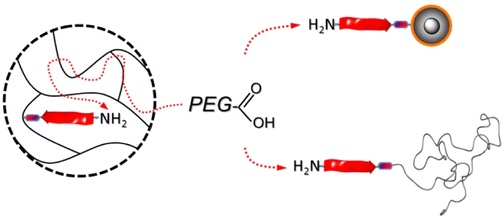
New Paper published in collaboration with Jean-François Lutz.
Efficient routes to N-terminal PEGylated peptides are described. Alternative supports such as superparamagnetic core–shell nanoparticles as colloidal supports and end-functional poly(styrene) as homogeneous supports improve the available solid-phase supported coupling strategies preserving ease of purification. Poly(ethylene glycol) (PEG)–peptide bioconjugates are obtained in high yield despite the use of nearly stoichiometric PEGylation agents with respect to the supported peptides.
PDF |
 |
|
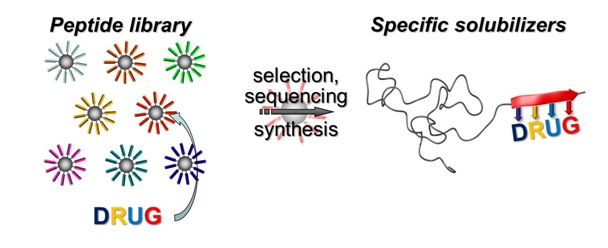
11.01.2013 - Exploiting Specific Interactions toward Next-Generation Polymeric Drug Transporters
|
New Paper in Journal of the American Chemical Society has been published.
A generic method describes advanced tailoring of polymer drug carriers based on polymer-block-peptides. Combinatorial means are used to select suitable peptide segments to specifically complex small-molecule drugs. The resulting specific drug formulation agents render insoluble drugs water-soluble and enable precise adjustment of drug-release profiles beyond established block-copolymer carriers.
PDF |
 |
|
13.08.2012 - (Bio)Molecular Surface Patterning by Phototriggered Oxime Ligation
New Paper published in collaboration with Christopher Barner-Kowollik (KIT).
Making light work of ligation: A novel method utilizes light for oxime ligation chemistry. A quantitative, low-energy photodeprotection generates aldehyde which subsequently reacts with aminooxy moieties. The spatial control allows patterning on surfaces (see scheme) with a fluoro marker and GRGSGR peptide and can be imaged by time-of-flight secondary-ion mass spectrometry.
English PDF German PDF |
molecular_surface_patterning.gif) |
|
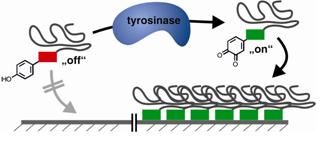
26.06.2012 - Mussel-Glue Derived Peptide–Polymer Conjugates to Realize Enzyme-Activated Antifouling Coatings
New Paper in ACS Macro Letters has been published.
Enzyme activated polymer coatings based on peptide-polymer conjugates are described. Tyrosinase is used to “switch on” adhesive functions of a mussel glue derived peptide segment, leading to bioconjugates that adhere to steel. The coating resists seawater treatments and modulates surface properties to anti-fouling surfaces by strongly reducing protein adsorption.
PDF
|
 |
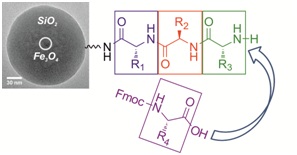
|
13.06.2012 - Superparamagnetic core-shell nanoparticles as solid supports for peptide synthesis
New Paper in Chem. Commun. has been published.
Magnetite nanoparticles are coated with a silica-shell and functionalized with amino groups to further introduce linkers moieties needed for peptide synthesis. The size of the colloidal supports of ~70 nm allows chemical peptide synthesis in “quasi solution”. An external magnetic field is used for magnetic sedimentation for ease of purification of the products after each reaction step.
PDF |
 |
|
08.05.2012 - Zimmer Award 2012
Hans Börner received:
International Hans and Marlies Zimmer Award 2012
from the University of Cincinnati, USA. |
 |
|
03.04.2012 - 2. Berliner Chemie Symposium (BCS) - AWARDS
2nd Berliner Chemie Symposium (BCS)
Patrick Wilke: Price for the second best Lecture held
Sebastian Wieczorek: Price for the best Poster submitted Congratulations!
|
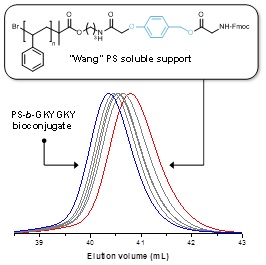
12.03.2012 - ‘‘Inverse’’ synthesis of polymer bioconjugates using soluble supports
Paper published in Chem. Commun. together with Dr. Jean-Francois Lutz (Strasbourg).
Well-defined cleavable or non-cleavable soluble polystyrene supports were prepared by atom transfer radical polymerization and utilized for the iterative synthesis of functional hexapeptides. This approach allowed rapid and efficient liquid phase synthesis of peptide–polymer conjugates.
PDF |
 |
|
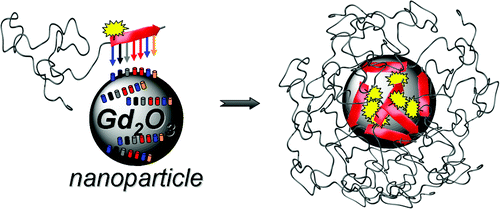
23.12.2011 - Peptide-mediated nanoengineering of inorganic particle surfaces: A general route toward surface functionalization via peptide adhesion domains.
New Paper in JACS has been accepted.
A one-step surface coating procedure is described. Coating is performed with peptide adhesion domains, found via phage display. This highly generalizable protocol permits various surface functionalization, which was shown by introduction of a fluorescent dye and PEGylation on Gadolinium oxide particles.
Manuscript ID: ja2104944; Journal of the American Chemical Society (JACS)
|
 |
|
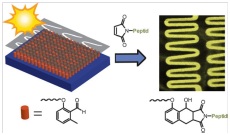
09.12.2011 - Adding Spatial Control to Click Chemistry: Photo triggered Diels–Alder Surface (Bio)functionalization at Ambient Temperature
New Paper published in collaboration with Christopher Barner-Kowollik (KIT)
A photoconjugation strategy based on light-triggered Diels–Alder addition of o-quinodimethanes is described. The method provides spatial control, is compatible with biomolecules, and proceeds rapidly at ambient temperature without the need of a catalyst.
DOI: 10.1002/anie.201107095, Angew. Chem. Intern. Ed. 2012
|
 |
|
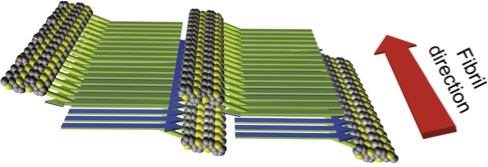
09.08.2011 - Self-assembling nanofibers from thiophene-peptide diblock oligomers: a combined experimental and computer simulations study
Paper in ACSNano has been accepted 08.08.2011
Bioconjugates combining PEO-functionalized peptide segments with covalently linked semiconducting alkylated quaterthiophene moieties are reported. Self-assembly and stimuli-responsiveness was investigated in a convergent experimental and computational approach.
|
 |
|
13.07.2011 - Berliner Tag der Chemie im Jahr der Chemie @ HU-Berlin
Der “Tag der Chemie” der Berliner Universitäten wird unter der Organisation des AK Börners an der HU-Berlin veranstaltet. Das diesjährige Motto “Studium....und dann?” soll zum Informationsaustausch zwischen Industrie und Universitäten anregen. Ausgewählte Beiträge beleuchten das Thema aus Sichtweisen namhafter Industrieunternehmen, Mittelständiger Unternehmen und Nachwuchsgruppen.
http://www.nordostchemie.de/tdc/tdc-tag-der-chemie
|
 |
|
15.06.2011 - A book was published on Bioactive surfaces
|
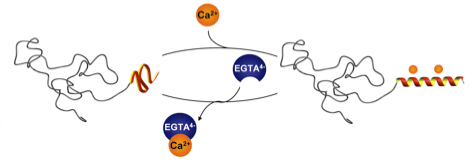
26.05.2011 - Calcium ions as bioinspired triggers to reversibly control the coil to helix transition in peptide-polymer conjugates
Paper to be published in Soft Matter A de novo designed calcium responsive peptide sequence was synthesized and the effect of conjugating poly (ethylene oxide) of different molecular weights on the calcium induced coil-to-helix transition was investigated. The secondary structure transition showed to be reversible upon addition of competitive calcium ion binders.
Soft Matter, DOI:10.1039/C1SM05625E
|
 |
|
08.04.2011 - Regulating hierarchical nanostructure formation in bioconjugates via calcium ions
Paper published in Angew. Chem. Int. Ed. Inter- and intramolecular Coulomb interactions of peptides are modulated leading to a biomimetic approach to reversibly regulate functions in peptide-polymer conjugates. As triggers to screen peptide charges and thereby switching the peptide secondary structure, calcium ions are exploited. This results in a reversible control mechanism for bioconjugate self-assembly. By regulating the Ca2+ levels using competitive Ca2+ binders disassembly is feasible.
ACIEE: DOI: 10.1002/anie.201100141 |
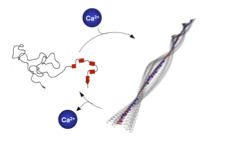 |
|
21.01.2011 - Watchmakers in Polymer Science – On the Road to Precision Macromolecular Chemistry
Special Issue published in Macromol. Rapid Commun. (vol. 32, issue 2, 2011) Guest-editors: Hans G. Börner (Humboldt-Universität zu Berlin) and Jean-Francois Lutz (Institut Charles Sadron, Strasbourg)
http://www.materialsviews.com/details/news/980719
|
 |
|
19.01.2011 - Precision Polymers — Modern Tools to Understand and Program Macromolecular Interactions
Paper published in Macromol. Rapid Commun. 2011, 32, 115-126
Peptide-block-polymer copolymers possess a monodisperse peptide segment with a freely programmable monomer sequence. An overview on the possibilities that arise from these precisely adjustable, functional polymers is presented.
http://onlinelibrary.wiley.com/doi/10.1002/marc.201000646/abstract |
 |
|
17.12.2010 - Single-step Electrospinning of Bioactive Polymer Nanofibers
| Polymer nanofiber meshes that possess biofunctional peptide segments on their surfaces are obtained with a standard electrospinning setup. Spinning a homogeneous mixture composed of a valuable polymer-peptide conjugate and a biocompatible commodity poly(ester) leads to nonwovens where the peptide part is enriched up to 11-times on the fiber surface. Bioavailability and bioactivity of the peptides are demonstrated as the meshes promote adhesion and migration of fibroblasts. |
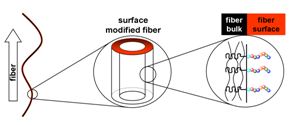 |
|
15.12.2010 - Merry X-mas - Wir wünschen allen frohe Weihnachten & ein erfolgreiches Jahr 2011!

@ Potsdam Weihnachtsmarkt |

@ Happy Sumo Weihnachts-Sushi |
|
17.11.2010 - Christian Stutz in den Bundesvorstand des JungChemikerForums gewählt
Christian Stutz vom Arbeitskreis Prof. Hans Börner der Humboldt-Universität zu Berlin wurde beim diesjährigen Sprechertreffen der JungChemiker in Würzburg in den Bundesvorstand des JungChemikerForums der Gesellschaft Deutscher Chemiker gewählt. Sein Ressourcengebiet umfasst die Öffentlichkeitsarbeit und Homepage des Forums.
www.jungchemikerforum.de |
 |
|
22.10.2010 - Modular Approach toward Bioactive Fiber Meshes Carrying Oligosaccharides
| In a single step, polymer nanofibers with pentafluorophenyl (Pfp) activated esters at the surface are fabricated by electrospinning of either pure poly(pentafluorophenyl methacrylate) (PPfpMA) or PPfpMA / poly(ε-caprolactone) (PCL) blends which can be successfully functionalized with amino-functional biomolecules like saccharides for immune response. |
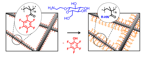 |
|
22.10.2010 - A modular approach towards functional decoration of peptide–polymer nanotapes
| Self-assembled peptide–polymer nanotapes of poly(ethylene oxide)–peptide conjugates are modified by a simple amine–azide transfer to create azide-containing nanofibres, which provide a platform for modular functionalization as demonstrated by the introduction of different carboxyl bearing entities to modulate the calcium binding properties of the nanotapes. |
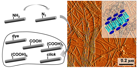 |
|